Doxepinone Doxepin Intermediate
Doxepinone Doxepin Intermediate Specification
- HS Code
- 29329990
- Molecular Formula
- C15H10O2N
- Loss on Drying
- 0.5%
- Boiling point
- Not available
- Particle Size
- Customizable, standard <100 micron
- Heavy Metal (%)
- 0.001%
- Residue on Ignition
- 0.1%
- Assay
- 98%
- Molecular Weight
- 238.27 g/mol
- Place of Origin
- India
- Storage
- Store in cool and dry place
- Moisture (%)
- 0.5%
- Acid Value
- Not determined
- Type
- Pharmaceutical Intermediate
- Grade
- Pharmaceutical Grade
- Usage
- Intermediate for synthesis of Doxepin
- Purity
- >98%
- Appearance
- Powder
- Application
- Raw material for Doxepin production
- Raw Material
- Benzene derivatives
- Smell
- Odorless
- Color
- Pale Yellow to White
- Form
- Solid
- Packing
- HDPE drums / fiber drums with double liner
- Density
- Approx. 1.25 g/cm³
- Impurity Level
- NMT 0.1%
- Country of Export
- India
- Stability
- Stable under recommended storage conditions
- Identification Method
- IR, NMR, HPLC
- Flash Point
- >200°C
- Storage Condition
- Protect from moisture and light
- Shelf Life
- 2 years if properly stored
- Solubility
- Slightly soluble in water, soluble in organic solvents
- Regulatory Compliance
- USP/EP standards if required
Doxepinone Doxepin Intermediate Trade Information
- Minimum Order Quantity
- 10 Kilograms
- Supply Ability
- 100 Kilograms Per Day
- Delivery Time
- 3 Days
- Main Export Market(s)
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- Main Domestic Market
- All India
About Doxepinone Doxepin Intermediate
We hold expertise in manufacturing, supplying and exporting Doxepinone. Having medicinal properties, these products are used for the treatment of used to treat depression and anxiety disorders. Highly effective in nature, these acids are processed under the guidance of chemical experts to leave no scope of defects. Demanded by pharmaceutical and chemical industries, the Doxepinone offered by us is available for our customers at industry leading prices.
Precision Manufacturing for Pharmaceutical Applications
Doxepinone is crafted under stringent quality controls, ensuring consistent pharmaceutical grade output. Known for its excellent purity (98%) and compliance with USP/EP standards upon request, it supports robust synthesis processes for Doxepin. As a trusted raw material, it meets industry requirements for impurity levels and moisture, making it a reliable choice for high-quality medication manufacturing.
Robust Stability and Safe Handling Features
With a stable formulation, Doxepinone boasts a shelf life of up to 2 years when protected from moisture and light. It comes packaged securely in HDPE or fiber drums with double liners to prevent contamination and preserve quality. Its high flash point (>200C) and customizable particle size further enhance storage safety and processing flexibility.
FAQs of Doxepinone Doxepin Intermediate:
Q: How should Doxepinone be stored to maintain its quality?
A: Doxepinone should be stored in a cool, dry location within well-sealed HDPE or fiber drums equipped with double liners. It is important to protect the product from moisture and light to retain its stability and shelf life.Q: What is the primary usage of Doxepinone?
A: Doxepinone is primarily used as an intermediate in the synthesis of Doxepin, an active pharmaceutical ingredient. Its high purity and quality make it suitable for pharmaceutical manufacturing processes.Q: When does Doxepinone expire and how long does it remain stable?
A: When properly stored under recommended conditions, Doxepinone has a shelf life of up to 2 years while maintaining its stability and integrity.Q: Where is Doxepinone manufactured and exported from?
A: Doxepinone is manufactured and exported from India, complying with international regulatory requirements such as USP and EP standards when necessary.Q: What identification methods are used to ensure the authenticity of Doxepinone?
A: Doxepinone is identified and verified through advanced analytical methods including Infrared (IR) spectroscopy, Nuclear Magnetic Resonance (NMR), and High-Performance Liquid Chromatography (HPLC), ensuring product quality and traceability.Q: How does the solubility of Doxepinone affect its application?
A: Doxepinone is slightly soluble in water but readily dissolves in organic solvents, making it suitable for chemical synthesis processes where mixtures in organic phases are often required.Q: What are the key benefits of using Doxepinone as a pharmaceutical intermediate?
A: The key benefits include high purity (>98%), low impurity levels, stable properties, compliance with major pharmacopeial standards, and customization in particle size, all contributing to higher efficiency and safety in pharmaceutical production.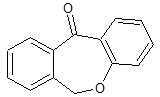

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Pharmaceutical Intermediates & Specialty Chemicals Category
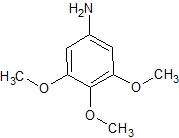
3 4 5-Trimethoxyaniline
Price Range 200.00 - 900.00 INR / Piece
Minimum Order Quantity : 10 Kilograms
Grade : Other, Industrial/Agricultural grade
Purity : >=98%
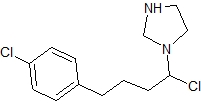
1- 2-Chloro-4-4-Chlorophenyl-Butyl-Imidazole
Price 200 INR / Kilograms
Minimum Order Quantity : 10 Kilograms
Grade : Medicine Grade
Purity : Greater than 99%
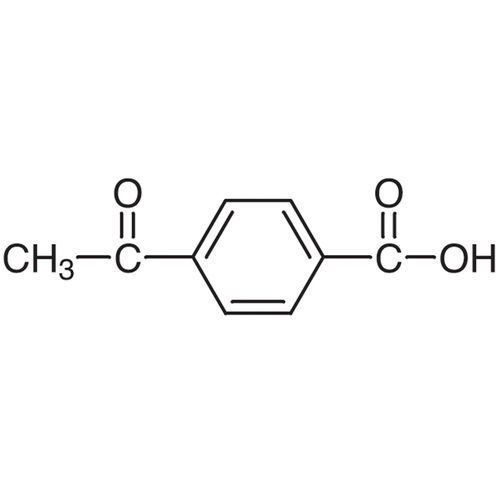
4-Acetybenzoic acid
Price 2500 INR / Kilograms
Minimum Order Quantity : 50 Kilograms
CAS No : 586890
N N-Diethylcyanoacetamide
Price 2000 INR / Kilograms
Minimum Order Quantity : 25 Kilograms
CAS No : 26391060

 Send Inquiry
Send Inquiry
 Send Inquiry
Send Inquiry Send SMS
Send SMS
